Toul Operio
Mobile Sterile Air Unit
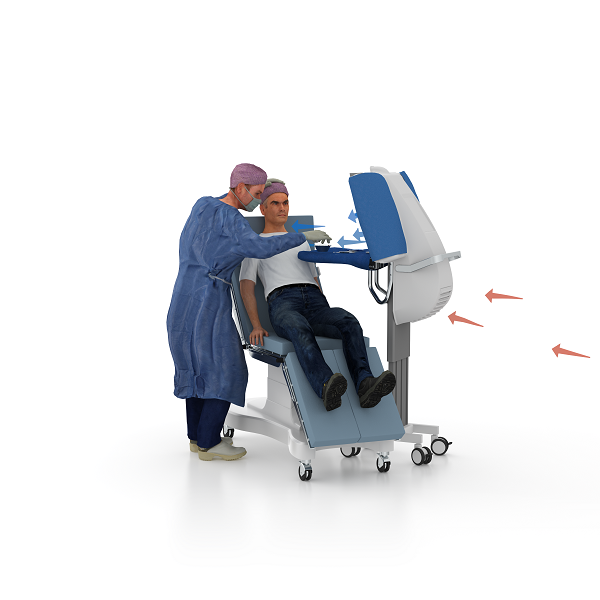
The innovative clean air zone unit that ensures both the surgical site and instruments near the wound remain sterile during the entire surgical procedure.
 Mobile sterile air zone unit
Mobile sterile air zone unit
 For use in the operating zone
For use in the operating zone
 Ideal for IVT injections
Ideal for IVT injections
Operio Sterile Air Unit
A smart alternative to keep your wound site sterile, the Toul Operio can be used in all types of operating rooms and surgical preparation rooms, independent of ventilation system. Toul Operio ensures that both the surgical site and instruments near the wound remain sterile during the entire surgical procedure.
Toul Operio generates additional IVT clinical capacity without affecting complication levels.
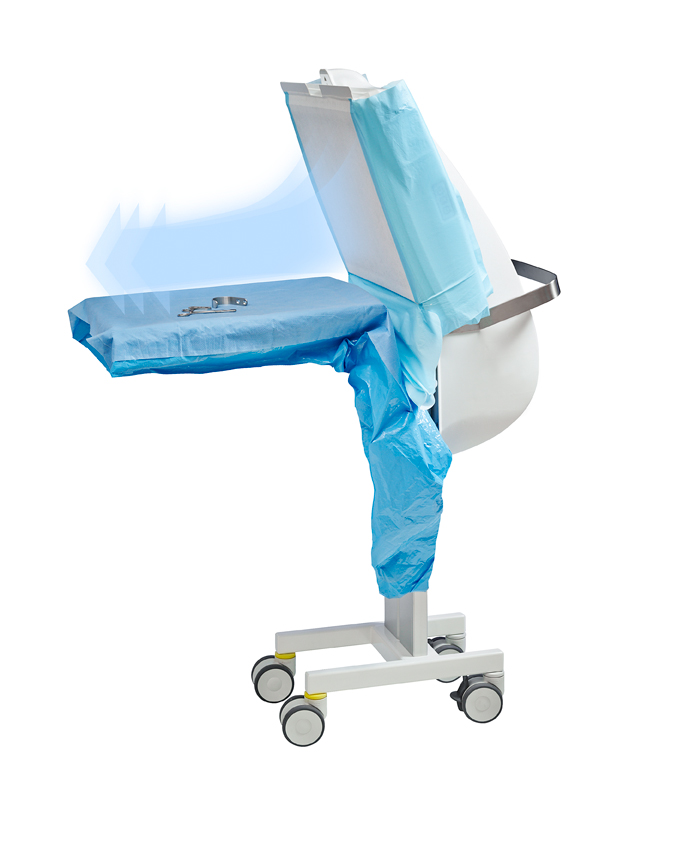
Sterile air zone
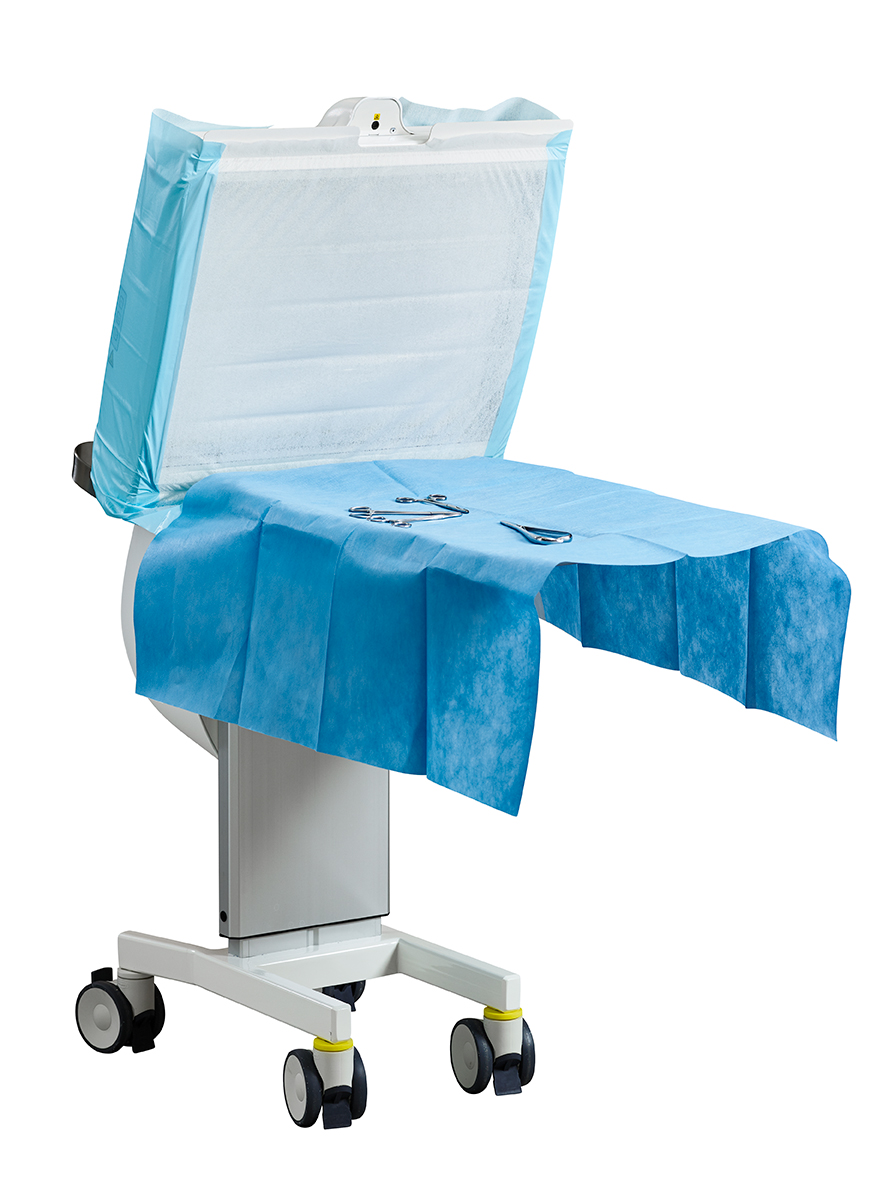
Guaranteed < 5 cfu/m3 air inside the clean air zone, with an integrated foldable instrument tray, directs non-turbulent ultra-clean air flow over the surgical site and/or sterile instruments. Toul Operio has a unique sterile protective barrier facilitating placement close to the operating table, saving valuable space and, can be used instead of an instrument table.
A smart alternative to keep your wound site sterile, the Toul Operio can be used in all types of operating rooms and surgical preparation rooms, independent of ventilation system. Toul Operio ensures that both the surgical site and instruments near the wound remain sterile during the entire surgical procedure.
Toul Operio has multiple uses:
- Protection of patient wound site
- Protection of surgical instruments
- Surgery: ophthalmic, ENT, maxillofacial including IVT injections, ocular plastics
- Increase of additional IVT clinical capacity
- Supporting critically ventilated rooms currently failing annual verifications
- Misappropriation of room classification e.g. treating sterile pack store (SPS) as lay-up preparation rooms or treatment rooms as operating rooms
HEPA filtration
Prevents dangerous, airborne bacteria-carrying particles from coming into contact with the surgical site, as well as nearby surgical instruments. The unit is easy to use and transport between operation rooms and/or preparation rooms.
Toul Operio ‘Clean air’
- Multi-setting usage: suitable for all types of operating and surgical preparation rooms, independent of ventilation system
- Ideal for IVT injections
- Multi-configuration: can be used instead of an instrument table near the wound next to the patient saving valuable space
- Guarantees < 5 cfu/m3 air inside the sterile air zone
Download
Images
Technical specifications
Brochures & Manuals
Description & Technical Specs
Overall size: 45 L x 60 W x 130 – 170 H cm
Weight: 42 kg
Tray size: 45 x 60 (optional 40 x 50)cm
Load limitation: Tray may be loaded with max 5 kg
Min-max height adjustment: 80 – 120 cm
Power supply: 230 VAC, 50 Hz
Power consumption: 290 VA (23 VA in standby-mode)
Air flow velocity: 0.4 – 0.5 m/s
Air cleaning capacity/hr: 400 m3/hr
HEPA Filter: H14 Filter, High Efficiency Particles Filter. Filters 99.995% particles >0.3 μm
Efficacy: Studies have shown a reduction from 48.2 to 0.4 Colony Forming Units in a clinical environment
Regulatory compliance: CE mark Class I according to Medical Device Directive 93/42/EEC